- Solutions
- Life Sciences
- Genetic Data Validation
Maximize Data and Minimize Risk With Genomic Data Validation
The right protocols ensure compliance without compromising clinical research
To maximize data and reduce the cost of clinical trials, the genomics experts at InformedDNA help biotech and pharmaceutical clients implement IRB-approved, compliant genomic data validation programs that maximize data for sponsors while protecting patients and ensuring compliance at every step.
Genomics Data Use and Compliance for Clinical Trials
As the number of personalized medicine therapeutics in clinical trials grows, sponsors and CROs face an increased need for genomic data and protocols for data compliance. The genomics experts at InformedDNA have over a decade of experience setting up genomic adjudication programs in collaboration with our biotech and pharmaceutical partners. We ensure that our clients’ clinical research programs meet ethical standards and comply with governance for data collection, storage, sharing, use, and reporting—without hindering researchers’ ability to utilize data effectively for clinical research.

Independent Consulting
Choose the most appropriate genetic test with unbiased guidance from a lab-agnostic genomics expert.

Informed Consent Protocols
Design informed consent protocols that enable research beyond the initial genetic test or clinical trial.

FDA Data Collection
Collect patient-reported outcomes and real-world evidence data for FDA submission.

Genetic Test Program Optimization
Gene panel testing program optimization to improve efficiencies and refine the research candidate pipeline.

Genomics Content Library
Meet guidelines for study documents that are appropriate for all reading levels, across diseases.
IRB Guidance
Ensure research protocols and related materials meet IRB guidelines and comply with data regulations.

Test Result Adjudication
Reduce the risk of secondary genomic variant impact prior to study enrollment.

VUS Resolution Support
Resolve VUS through family member testing and collaboration with a broad network of lab partners.

Data Augmentation
Access additional clinical data for genotype/phenotype analysis with records review and patient outreach.
200,000
8,000
5-30 yrs
Let’s talk about your needs!
Thank you for considering InformedDNA as your applied genomics solutions company. Please let us know how we can help meet your needs.
Please do not include any protected health information (PHI) in your message to us.
Required Information*
Applied Genomics Resources and Expertise
~50%
of all therapies in clinical trials are collecting biomarker and genomic data; that number rises to 73% for oncology trials
$30,000
average cost
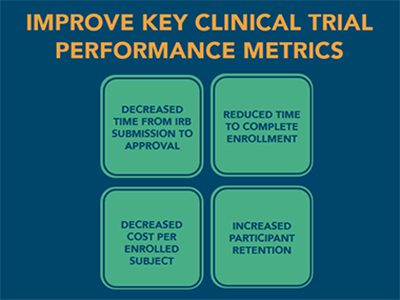
Genomic Data Validation Protocols Streamline the Clinical Trial Process
Genomic components add many intricacies to a clinical trial. In some instances, panel tests must be large enough to meet the needs of a patient population, but avoid unnecessary genes that return clinically irrelevant data. At other times, a test must be broad enough to capture data that might drive future trials. Our rare disease and subspecialty genomics expertise and genomic data validation protocols help InformedDNA clients:
- Identify clinical trial candidates
- Choose appropriate genetic tests
- Customize genetic tests to reduce VUS
- Comply with state and national regulations
Experienced Genetic Counselors Are Partners in Data Validation
Genetic testing in the context of clinical trials raises important ethical issues related to data privacy, informed consent and disclosure of results, all of which are central to genomic data collection and validation. Our expertise in rare disease genomics makes genomic trials more efficient, patient-centered and, ultimately, successful through:
- Genomic data validation protocols
- Detailed medical and family histories
- Patient experience data and insights
- Informed consent protocols
Data Compliance Can Make or Break Clinical Research
With numerous national regulations and standards regarding genetic testing, informed consent and results disclosure, and genomic data use in clinical trials, it is important to know how data can and should be used to mitigate risk for sponsors. Regulations governing patient data include:
- Health Insurance Portability and Accountability Act of 1996 (HIPAA)
- Genomic Information Nondiscrimination Act (GINA)
- Affordable Care Act (ACA)
- Americans with Disabilities Act (ADA)
- State laws
- Institutional Review Board (IRB) guidelines
- FDA regulations
Penalties for misusing or not protecting patient data can be steep, and “I wasn’t aware” is not a viable defense. InformedDNA’s full-time genomic specialist staff—the nation’s largest and most experienced—can be leveraged to create IRB-approved, compliant programs that maximize data for sponsors while protecting patients and ensuring adherence to data regulations at every step.
We help sponsors develop and implement genomic screening programs that meet national guidelines for data collection, storage, dissemination, and use for current and future clinical research. We also guide the design of protocols to ensure that all genomic information is appropriately applied to study design.
Our Latest Thinking on Genomics
- How HealthCare Providers Can Bolster Patient Medication Safety this National Check your Meds Day 2023
Your Trusted Partner in Applying Genomic Science
InformedDNA is the nation’s first telehealth genomics services company. Our real-world clinical guidance, cost management and patient navigation solutions are built upon the most current genomics insights and are designed to optimize clinical decisions across the care delivery spectrum.
We have helped manage the health benefits of more than 100 million covered lives and have navigated hundreds of thousands of people to the right treatments or clinical trials.